
CPI selects Suncombe Effluent Treatment Facility for UK's new National Biologics Manufacturing Centre
The Centre for Process Innovation (CPI) has recently completed commissioning of the new £38m National Biologics Manufacturing Centre in Darlington, Co. Durham, which features a state-of-the-art effluent treatment facility suitable for Containment Level 2 wastes, supplied by Suncombe’s Biowaste Solutions arm.
 National Biologics Manufacturing Centre
National Biologics Manufacturing Centre
The new Centre provides companies with open access facilities and expertise to help them develop, prove and commercialise new and improved processes and technologies for biologics manufacture. The facility has a limit of Category II containment. So the waste liquid needs to be collected and thermally denatured prior to discharge to the drain.
The flagship National Biologics Centre is part of the UK Governments Strategy for Life Science, helping UK companies to develop a competitive foothold in the growing global biopharmaceutical market. Biological medicines already account for around 10 – 15% of the current pharmaceutical market and the sector is outperforming the market as a whole. More than one-fifth of new medicines launched on the world market each year are now biotechnology-derived.
The Suncombe equipment is based on its EDS+ Effluent Decontamination System range, with additional features developed to suit the client’s specific requirements. This included Suncombe engineers being part of a team which constructed a 3D model of the proposed Centre, which integrated with the sites overall layout, to allow visualisation of the precise location and position of the treatment facility.
 EDS+ Effluent Decontamination System
EDS+ Effluent Decontamination System
Steve Overton, technical director at Suncombe, commented, “We were delighted to be part of this project which is developing new, high-tech manufacturing businesses for the UK. As British manufacturers ourselves it is also important to be able to show off the expertise in biowaste engineering which is available in this country.”
“The team we were a part of, particularly the CPI staff, made this a very rewarding and exciting project to work on. Everyone came together very quickly to deliver a highly complex facility on schedule and to specification,” added Steve.
The project scope included the connection of user points (sinks) and a collection sump to the collection pipework, plus the design and modelling of this pipework, in stainless steel from the laboratories to the EDS+ system. It included a number of technical advances, including different user-level login facilities, variable treatment temperatures and times, anti-foam control, pH neutralisation, out-of-hours cooling methodology and 100 per cent positive release for treated waste. Another feature was emergency discharge to a road tanker.
Extensive pre-delivery tests were carried out to ensure that, on delivery to site, the system could be reconnected and ready for operation without any hitches. Factory Acceptance Tests (FATs) included system documentation and certifications reviews, as well as full testing, including automation and dry run simulations, wet testing and system thermal inactivation cycles.
Fergal O’Brien, Director of the National Biologics Manufacturing Centre said, “The new facility will support the development of new innovative process technologies and manufacturing routes. We will provide both large and small companies with open access facilities to prove and scale up their process, therefore reducing risk associated with product development.”
“Given the open access and variety of biologics on test it is vitally important that the effluent treatment facilities are able to cope with a range of products and remain fully compliant with the Category II containment requirements. The Suncombe system met our challenging specifications and is working well,” he added.
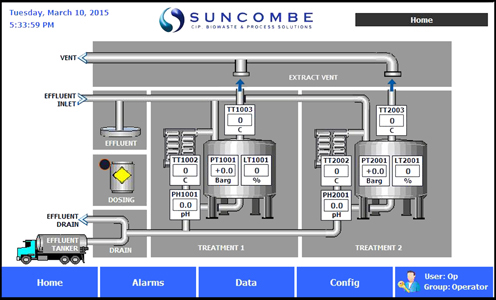 Operator Interface
Operator Interface

