
Sterile Filtration System for GSK Ulverston, UK
Suncombe meets challenge of automating sterile filtration system
New skid design improves sterile manufacturing and safety at pharmaceutical facility.
The GlaxoSmithKline (GSK) pharmaceutical manufacturing facility in Ulverston, United Kingdom had a manual sterile filtration system used to transfer product into a Grade C clean room. The company required a more automated solution and Suncombe became part of an Award Winning team which set out to meet the challenges of this exciting and complex project and successfully turn a design into a working system.
Typically, filter integrity testing involves a number of manual operations and relies on a path to atmosphere to test integrity. The GSK setup consisted of two filters and interchangeable hose connections which were operated manually.
This configuration did not protect the sterile nature of the filtration system, as exposure to the environment was inevitable during operation. The client required a new system which was fully integrated and completely automated, whilst being capable of protecting the sterile boundary during filter integrity testing.
So GSK’s challenge was to design and build an automated sterile filtration skid with hard-piped, integrated integrity testing, and no potential routes for contamination during post-sterilisation, integrity testing, and pre-use. Only the change of the filter element would remain as a manual operation.
Objectives
GSK assembled a strong team of specialists, comprising Suncombe as the preferred mechanical design team and subsequent fabricator, valve manufacturer ITT Engineered Valves and design, construction and commissioning experts PM Group; as well as senior personnel from the Ulverston facility. From the outset there were clear operational objectives for the resulting skid design.
These objectives were:
- To minimize the amount of equipment inside the clean room
- Automation of the integrity testing, operation, Clean-in-Place (CIP), and Steam-in-Place (SIP) to eliminate risks of manual operations
- Remove the risk of contamination through open ends
- Allow for dual filtration and multiple inlets and outlets
Suncombe, GSK and ITT Engineered Valves worked to design the filtration skid. During the design sessions, the team discussed system requirements and constraints. Using a piping and instrumentation diagram (P&ID), they examined slopes, dead-legs (where fluid can become stagnant) and overall height so that all aseptic design criteria were met.
The design had to allow for off-site fabrication, testing and validation to reduce construction activities.
Technical Approach
Having been chosen as the preferred mechanical design team and fabricator Suncombe attended the initial meeting to kick-off of the project. Rather than a typical ‘meet-and-greet’, this meeting involved all key-stakeholders and major equipment manufacturers, allowing for meaningful design discussion from day one.
Once the P&ID had been constructed, reviewed and approved by all parties, the detailed 3D design started. Weekly meetings, regular and frequent teleconferences and mutual site visits allowed project progress to be monitored closely, and feedback from all parties could be incorporated as appropriate.
Collaborative working ensured that all elements of the proposed system satisfied the design intent, and over a number of iterations, a final design evolved incorporating compliant line gradients, minimized line dead-legs and millimetre-tolerances over the entire system.

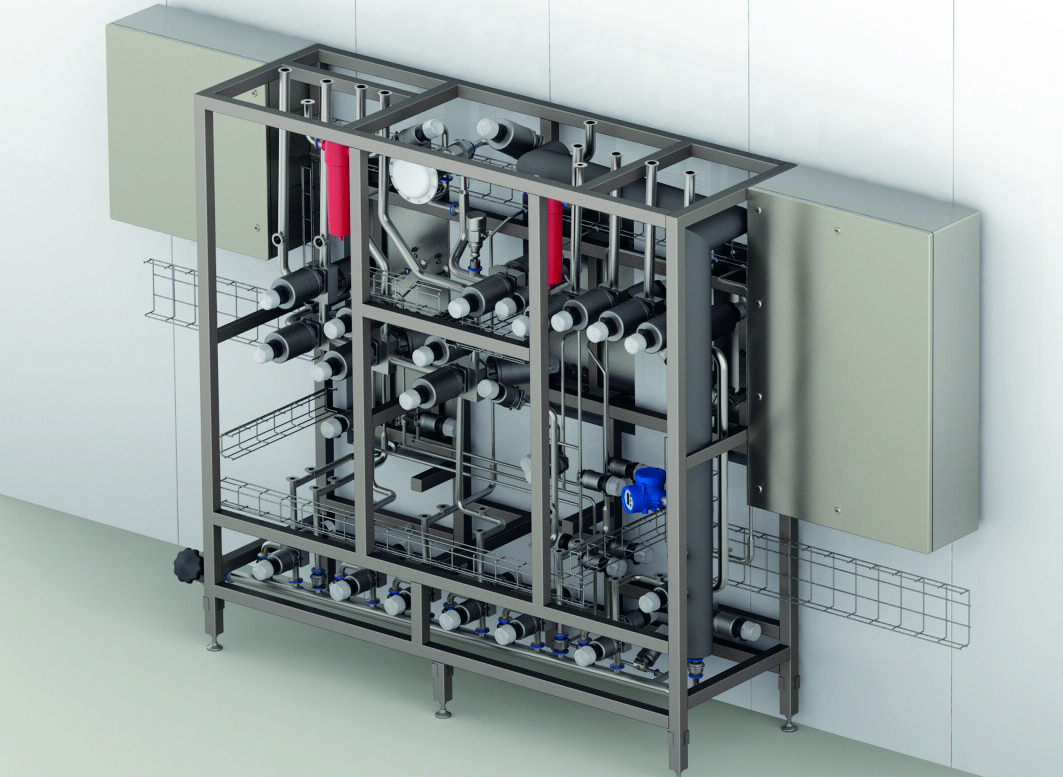

Over a period of six months, a system was developed which comprised of over 70 valves, including multiple mono-block valves, to allow repeatable inlet/outlet configurations for maximum compatibility as well as optimizing the efficiency of the process and reducing the resources required to produce a batch.
The skid permitted dual filtration, by having each filter connected via permanent pipework to the integrity tester, which is controlled by the Plant Control System. Any open ends were protected by additional filter units (which can also be integrity tested), or by a flow-controlling operational pressure regime.

Integration
The filtration skid was designed to integrate with a clean room, but keep a majority of the valves in a technical area outside the clean room. As the system is fully automated, this allows the plant control system to utilize 55 different operating sequences.
Due to the complete integration with the clean room walling system, a degree of flexibility of positioning was required whilst maintaining a hygienic barrier between the ‘clean’ point-of-use equipment, and the technical area valves/pipework/etc. behind the walling.
Manual operation is limited to essential procedures only (filter changes). While the unit allows for multiple inlets (multiple process streams as well as Pure Steam, WFI, Compressed Air, N2) and multiple outlets to a number of production vessels. Also the design had to meet several regulatory requirements: ATEX (as solvents are being filtered); ASME BPE; and current GEP practices with regards to safety (process and operational safety).
Challenges Met
Steve Overton, technical director at Suncombe, commented, “We were delighted to be part of the team for this prestigious project within GSK. Using our 50+ years of experience of producing hygienic pharmaceutical systems, and working with Glaxo Smith Kline, PM Group and ITT, the team provided a state-of-the-art system. The project provided great challenges for the whole team and I am proud to say that these challenges were met and exceeded.”
Award
At the IChemE Global Awards Ceremony for 2018, which was held in November in Manchester, Suncombe – along with the client and other project partners – was delighted to be announced as the winning entry for the Pharma category. The evening celebrated excellence, innovation and achievement in the chemical, biochemical and process industries.

Alun Cochrane, IChemE Award Host presenting the Pharma Award 2018 to the winnng team: GSK, ITT, PM Group and Suncombe for “Fully Integrated Sterile Filtration Unit”
Benefits
As well as reducing risks within the production process and enhancing the ability of the facility to maintain production and quality, the design and automation of the filter skids will allow the operation of the process stream to be optimized. This will improve the overall efficiency and cost effectiveness of the process. In addition operational safety will be greatly enhanced, with full containment of harmful fluids and gases and almost complete removal of manual operations.
From an environmental perspective, as the process will be improved, optimised and made more efficient, the volumes of processing fluids/utilities required, and the volumes of effluent and waste produced will be reduced, lowering the site’s overall energy and utility resource requirements.
Since the filter system will be used in a sterile manufacturing environment, this design will enable GSK to maintain the highest standard of product to its patients, whilst providing its staff with a greatly improved manufacturing environment.


